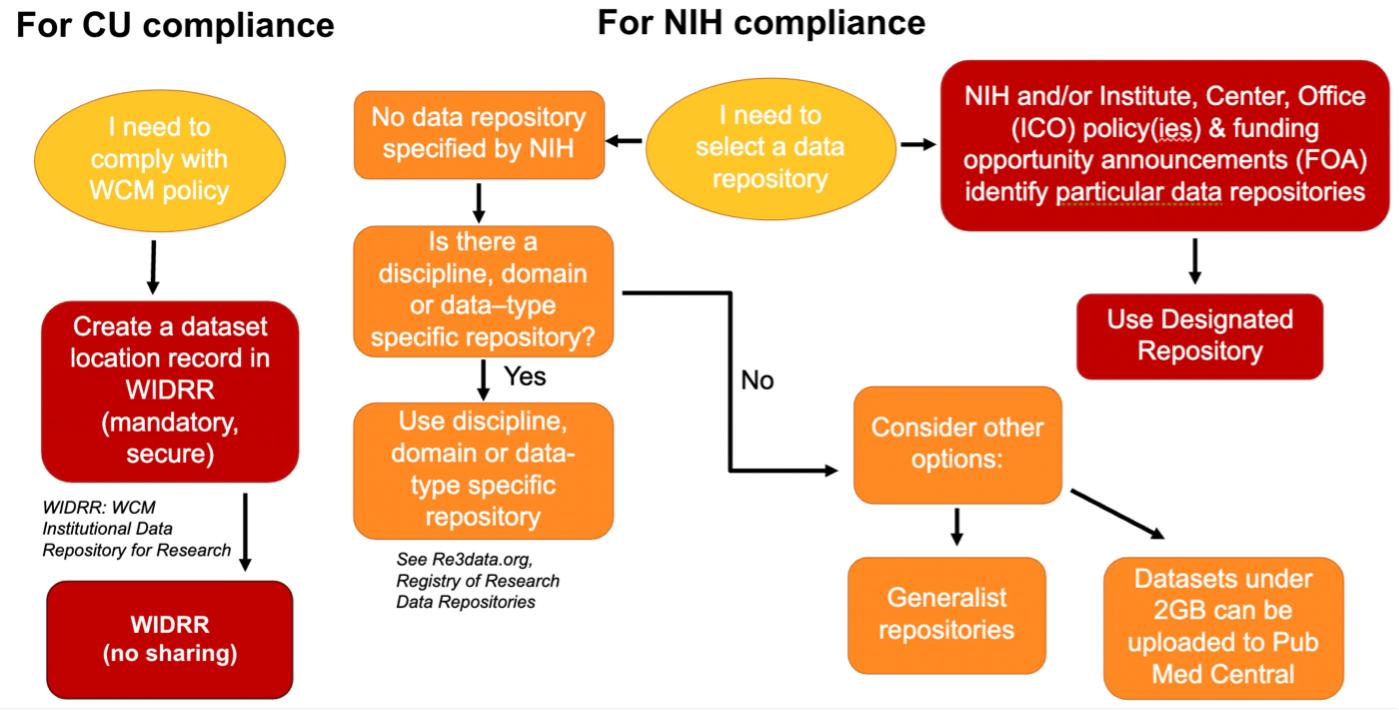
Who has access to the Samuel J. Wood Library?
All students, faculty, and staff of Weill Cornell Medical College and Weill Cornell Graduate School of Medical Sciences, as well as all staff of NewYork-Presbyterian Hospital and affiliated institutions, have access to the Library.
The Library is not open to the general public or to patients of NewYork-Presbyterian Hospital or their families.
Alumni of the Medical College must show a letter of verification from the Office of Alumni Relations for access to the Library.
Alumni of the Graduate School must present a letter of verification from the Graduate School Office. A separate Library card will be issued to eligible alumni at that time.
Members of the Center Alumni Council must show a current membership card.
Alumni of Cornell Ithaca must register at the circulation desk.


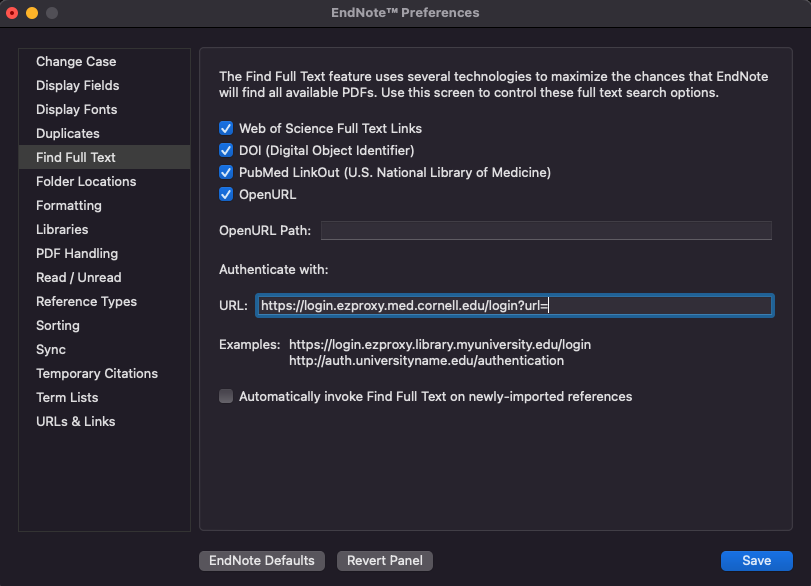
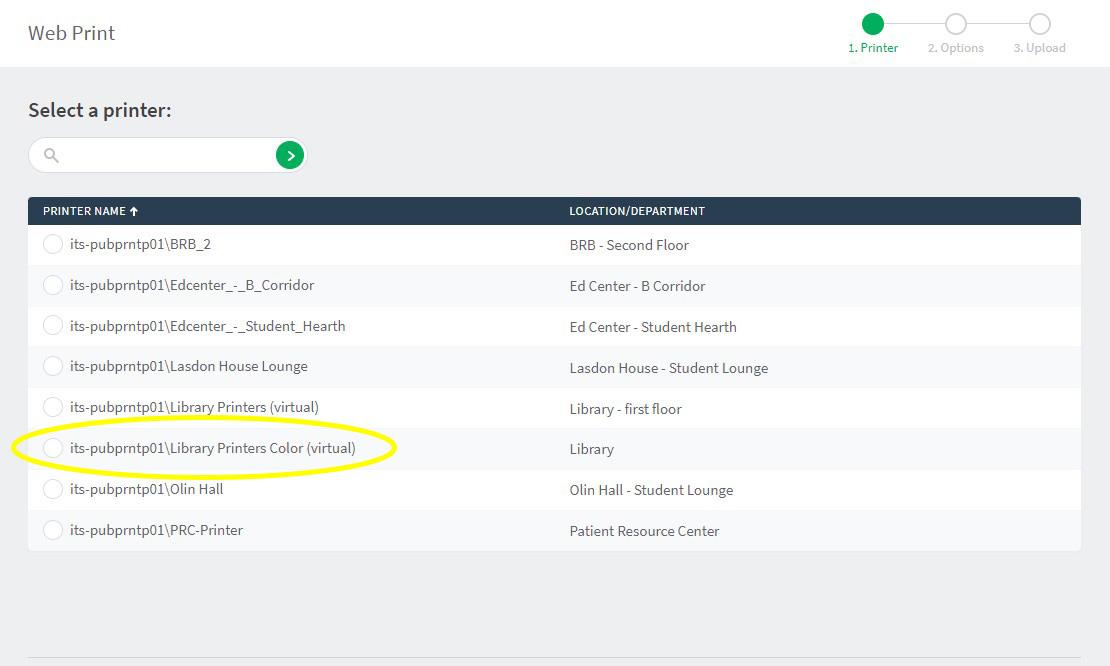

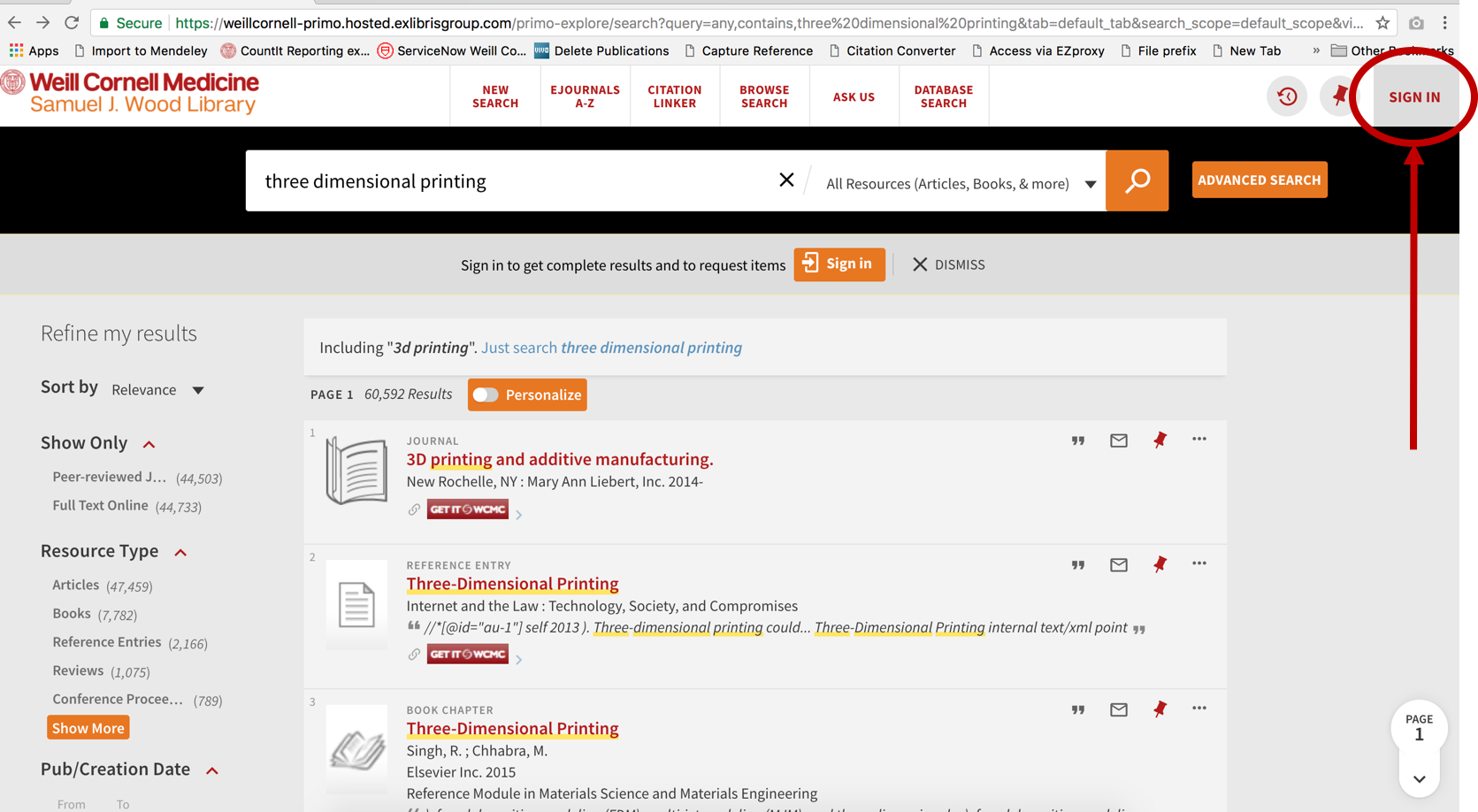
 if desired
if desired